Methane Combustion CFD Simulation: NOx Formation Analysis with Excess Air in ANSYS Fluent
Methane Combustion CFD Simulation: NOx Formation Analysis with Excess Air in ANSYS Fluent
- Upon ordering this product, you will be provided with a geometry file, a mesh file, and an in-depth Training Video that offers a step-by-step training on the simulation process.
- For any more inquiries regarding the product, please do not hesitate to reach out to us at info@CFDLAND.com or through our online support assistant.
€69
Burning methane (CH4) is a common way to generate energy. When methane mixes with oxygen (O2), it creates Carbon Dioxide (CO2), Water Vapor (H2O), and a lot of heat. This is called an exothermic reaction. In real industrial machines, we rarely use the exact amount of air needed. Instead, we use “excess air” to make sure all the fuel burns completely.
In this Methane Combustion CFD simulation, we model a specific case with 28% excess air. The chemical equation changes because of this extra air. We include Nitrogen (N2) in the reaction because it leads to pollution. The goal of this ANSYS Fluent training is to predict the formation of Nitrogen Oxide (NOx). NOx is a harmful pollutant, and simulating it helps engineers design cleaner burners.
CH4 + 2.56O2 + 9.6256N2 ® CO2 + 2H2O + 0.56O2 + 9.6256N2
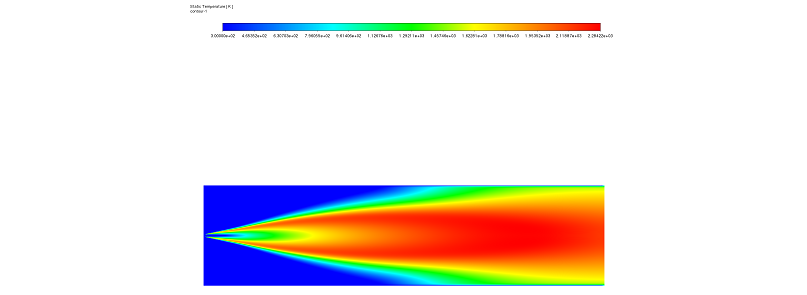
Figure 1: Temperature distribution contour showing high heat release near the burner.
Simulation Process: Modeling Methane Combustion and NOx Formation with Fluent
To start this CFD analysis, we modeled a cylindrical combustor. The geometry is designed for a high-speed jet. Methane is injected into the center and expands without hitting the outer walls too quickly. It mixes with the low-speed air surrounding it. To simulate this accurately, we generated a “structured mesh grid.” This grid covers the whole combustor and consists of exactly 34,100 elements. A structured grid is very important because it helps calculate the flow direction precisely.
For the physics in ANSYS Fluent, we selected the Species Transport model. This model allows the software to track different gases like Methane, Oxygen, and Carbon Dioxide separately. Because the flow is turbulent and reacts chemically, we used the Eddy Dissipation model. This model calculates how the turbulence mixes the fuel and air before they burn. Furthermore, to capture the pollution data, we enabled both Prompt and Thermal NOx formation models. This ensures we see all types of nitrogen oxides created during the heat release.
Post-processing: NOx Formation and Velocity Analysis
In this section, we analyze the results of the Methane Combustion CFD. We look at the connection between heat, speed, and pollution. First, the Temperature Contour (Figure 1) gives us a clear view of the flame. We can see areas of very high temperature located right next to the burner. This indicates the “ignition source” where the reaction starts. As the methane burns, the Mass Fraction of the species changes. At the inlet, the methane concentration is high. However, as it moves downstream, the methane disappears. It is replaced by combustion byproducts like CO2 and H2O. Simultaneously, at these high temperatures, the Nitrogen reacts with Oxygen to form NOx (Nitric Oxide and Nitrogen Dioxide).

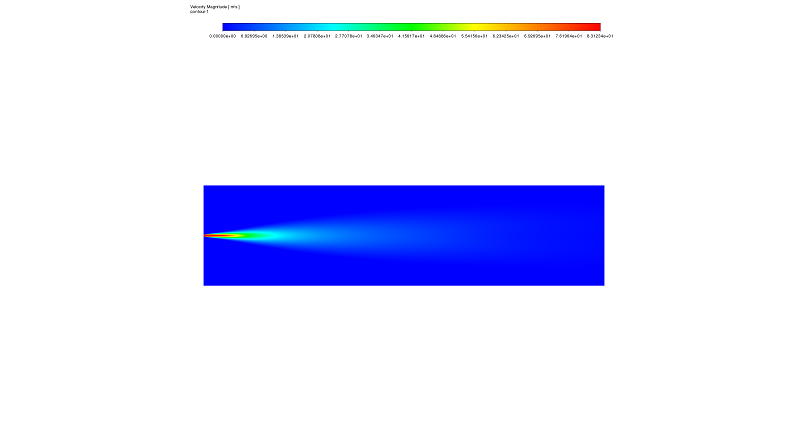
Figure 2 & 3: NOx mass fraction from the NoX Formation CFD analysis, highlighting the regions of high pollutant concentration – Velocity field from the Methane Combustion with excess air CFD simulation, showing the central jet and recirculation zones that influence NOx formation.
Second, we observe a critical link between the Velocity Field and NOx Production. The velocity contour (Figure 3) shows a typical jet pattern. The core of the jet moves very fast, but as it goes further, it slows down. This creates “recirculation zones” on the sides where the air moves slowly and mixes. This flow pattern directly changes the pollution levels. Consequently, the NOx Concentration Contour (Figure 2) shows that the highest pollution is not in the fast jet, but in the “post-flame zone.” This happens because of a concept called “residence time.” In the central jet, the gas moves too fast to react fully with nitrogen. However, in the slow peripheral regions, the hot gas stays for a longer time. This long “residence duration” combined with high temperature creates the perfect condition for Thermal NOx formation via the Zeldovich mechanism. This analysis proves that to reduce pollution, we must control both the temperature and the speed of the gas mixture.
We pride ourselves on presenting unique products at CFDLAND. We stand out for our scientific rigor and validity. Our products are not based on guesswork or theoretical assumptions like many others. Instead, most of our products are validated using experimental or numerical data from valued scientific journals. Even if direct validation isn’t possible, we build our models and assumptions on the latest research, typically using reference articles to approximate reality.
Yes, we’ll be here . If you have trouble loading files, having technical problems, or have any questions about how to use our products, our technical support team is here to help.
You can load geometry and mesh files, as well as case and data files, using any version of ANSYS Fluent.
€380 Original price was: €380.€185Current price is: €185.

€240 Original price was: €240.€125Current price is: €125.

€255 Original price was: €255.€135Current price is: €135.

€360 Original price was: €360.€185Current price is: €185.

€200 Original price was: €200.€115Current price is: €115.





















Reviews
There are no reviews yet.