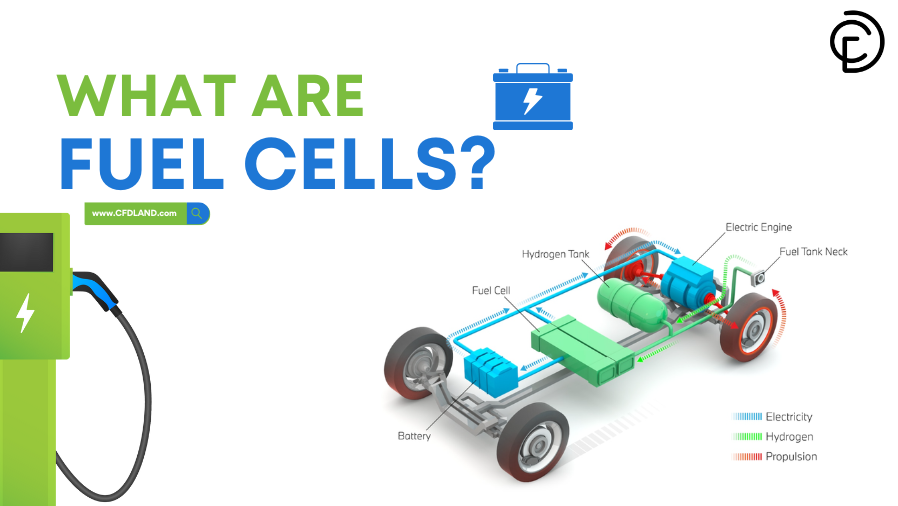
Fuel Cells are cutting-edge electrochemical devices that convert chemical energy directly into electrical energy. Unlike combustion-based systems, they offer high efficiency and low emissions, making them a promising solution for clean energy generation and sustainable energy solution. This blog explores the definition of fuel cells, their working principles, types, uses, and advantages compared to batteries. Key concepts relevant to A-level chemistry, along with detailed fuel cells diagrams, are discussed to provide insights into their applications. Whether you are interested in hydrogen fuel cells, their efficiency, or how hydrogen fuel cell cars work, this article is designed to give you a comprehensive understanding of fuel cells. At CFD LAND, we also explore how fuel cells are simulated using ANSYS Fluent to better understand and optimize their performance.
Contents
ToggleWhat are Fuel Cells?
Fuel cells meaning refers to devices that generate electricity through a chemical reaction between a fuels—typically hydrogen—and an oxidizing agent, such as oxygen. The key reaction occurs without combustion, allowing fuel cells to operate quietly and efficiently. In simple terms, the fuel cells definition describes a clean energy converter that can power everything from vehicles to power plants.
How do Fuel Cells Work?
The figure 1 illustrates the working principle of a fuel cell (fuel cells chemistry), broken down into the following key steps:
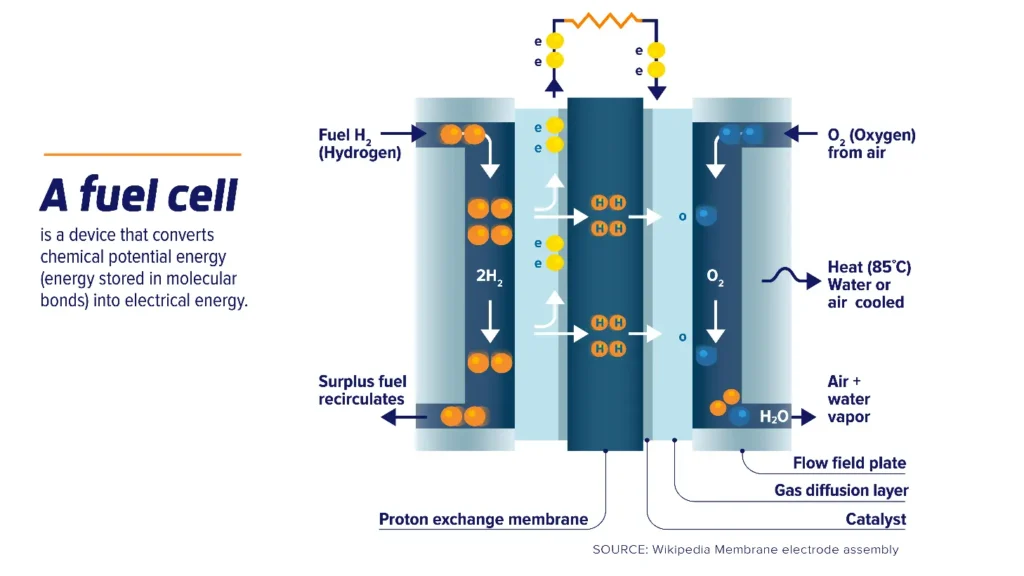
Figure 1- Schematic Representation of Proton Exchange Membrane Fuel Cell (PEMFC) Working Principle [1]
- Hydrogen Fuel Input (Anode Side):
As shown in the fuel cell diagram, Hydrogen gas (H₂) is supplied to the anode side, where it interacts with a catalyst layer. The hydrogen molecules are split into protons (H⁺) and electrons (e⁻) by the catalyst. This process is called the electrochemical oxidation:
H₂ → 2H⁺ + 2e⁻
Then, the protons (H⁺) travel through the Proton Exchange Membrane (PEM) toward the cathode side.
2.Electron Flow (External Circuit):
As illustrated in the fuel cell diagram, the electrons (e⁻) cannot pass through the membrane and instead flow through an external circuit, generating an electric current that can power devices. This flow of electrons is the electricity produced by the fuel cell.
3. Oxygen Input (Cathode Side)
Oxygen gas (O₂), typically from air, is fed to the cathode side of the fuel cell. At the cathode, oxygen reacts with the protons (H⁺) that have crossed the membrane and the incoming electrons (e⁻) from the external circuit, forming water (H₂O) as a byproduct:
O₂ + 4H⁺ + 4e⁻ → 2H₂O
4. Heat and Water Production
The electrochemical reaction produces heat (typically around 85°C) and water vapor as byproducts. Excess heat is managed through air or water cooling systems integrated into the fuel cell. Generally, the fuel cell achieves the following net reaction:
H₂ + O₂ → H₂O + Electricity + Heat
Key Components:
- Proton Exchange Membrane (PEM):A thin, permeable layer that allows protons (H⁺) to pass while blocking electrons (e⁻).
- Catalyst Layer:Facilitates the chemical reactions at both the anode and cathode.
- Gas Diffusion Layer & Flow Field Plate:Ensure uniform distribution of gases and aid in the removal of water formed during the reaction.
Types of Fuel Cells
There are several types of fuel cells, each designed for different uses. As technology evolves, we are seeing more fuel cells for sale across various industries. Fuel cells have also attracted much attention due to its environment friendly nature compared to the conventional generators, which generate harmful gases as by-products. According to table 1, different types of fuel can be used to drive the fuel cells. Pure hydrogen is commonly used by low-temperature fuel cells such as alkaline fuel cell (AFC) and polymer electrolyte membrane fuel cell (PEMFC). The pure hydrogen itself can be produced from hydrocarbons, methanol or syngas. High-temperature types such as molten carbonate fuel cell (MCFC) and solid oxide fuel cell (SOFC) are more flexible in the use of the fuel.
Table 1- Comparison between different type of fuel cell [2]
| Fuel Cell Type | PEMFC | AFC | DMFC | PAFC | MCFC | SOFC |
| Operating temp (°C) | 30–100 | 90–100 | 50–100 | 160–220 | 600–700 | 500–1000 |
| Electrical efficiency (%) | 30–40 | 60 | 20–25 | 40–42 | 43–47 | 50–60 |
| Energy conversion efficiency (%) | 85–90 | 85 | 85 | 85–90 | 85 | up to 90 |
| Typical stack size | <1–100 kW | 10–100 kW | Up to 1.5 kW | 50–1000 kW (250 kW module typical) | <1–1000 kW (250 kW module typical) | 5–3000 kW |
| Electrolyte | Solid polymeric membrane | Aqueous solution of potassium hydroxide soaked in a matrix | Solid organic polymer poly-perfluoro sulfonic acid | 100% phosphoric acid stabilized in an alumina-based matrix | Li2CO3/K2CO3 materials stabilized in an alumina-based matrix | Solid, stabilized zirconia ceramic matrix with free oxide ions |
| Fuels | Hydrocarbons or methanol | Pure hydrogen | Methanol | Hydrogen from natural gas | Natural gas, biogas, others | Natural gas or propane, hydrocarbons or methanol |
| Operational life cycle | 40,000–50,000 h | Up to 5000 h | 10,000–20,000 h | Up to 40,000 h | Up to 15,000 h | Up to 40,000 h |
Fuels Cells have potential commercialization applications. Figure 2 shows the power-delivering capability of various Fuels Cells applications.
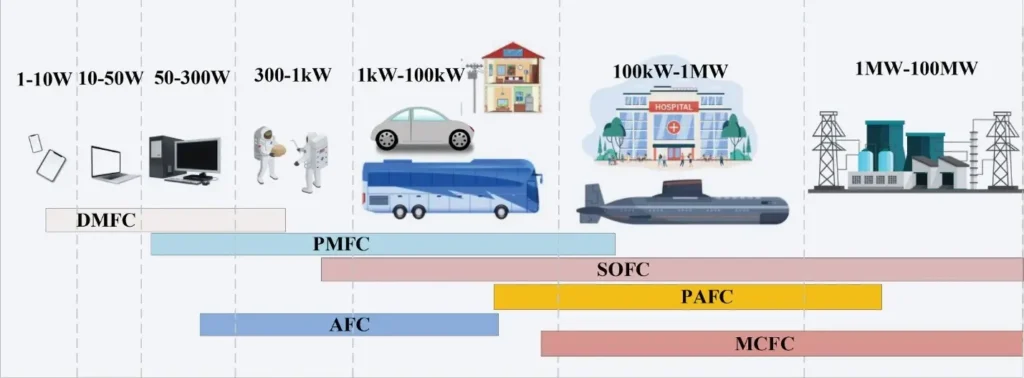
Figure 2- Power levels of portable and stationary Fuel Cells uses [3]
Figure 3 also compares different types of fuel cells based on their electrolytes, operating temperatures, and ion transfer mechanisms between the anode and cathode.
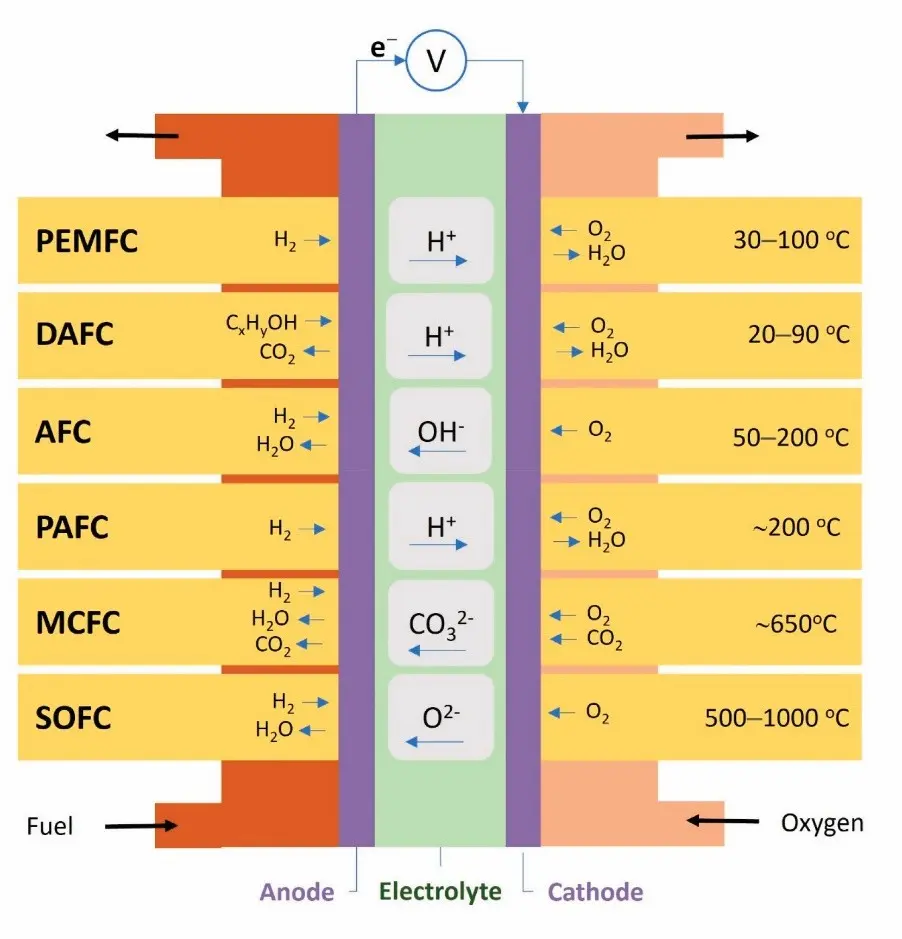
Figure 3- Classification of Fuel Cells examples based on the fuel and electrolyte [4]
Fuel Cells vs Batteries
Understanding the differences between fuel cells vs batteries (fig.4) is key to evaluating which technology suits a specific application. Although both systems are electrochemical devices that generate electricity, their operation, efficiency, and applications differ significantly.
Figure 4- Fuel Cells vs Batteries
Batteries
- Store energy chemically and release it through redox reactions.
- Require recharging once depleted.
- Have limited energy capacity based on size.
- Common in portable electronics, electric vehicles, and backup power systems.
Fuel Cells
- Continuously produce electricity as long as fuel (e.g., hydrogen) is supplied.
- Do not need recharging—just refueling.
- Typically higher efficiency for long-duration energy needs.
- Ideal for hydrogen fuel cell cars, industrial applications, and clean power systems.
The figure 5 showcases a fuel cell stack, which is a collection of individual fuel cells assembled to generate electrical power. Each cell within the stack contains an anode, cathode, and electrolyte, working together to convert chemical energy from hydrogen and oxygen into electricity. The metallic design indicates durability and precision engineering, while the visible connectors and gauges suggest features for monitoring and integrating the stack into larger systems, such as vehicles or stationary power units.

Figure 5-A Fuel Cell Stack
Hydrogen Fuel Cell Cars: How it Works
Hydrogen fuel cell cars (Fig.6) use fuel cells to generate electricity through an electrochemical reaction between hydrogen (stored in high-pressure tanks) and oxygen from the air, producing electricity to power the electric motor, with water and heat as the only byproducts.
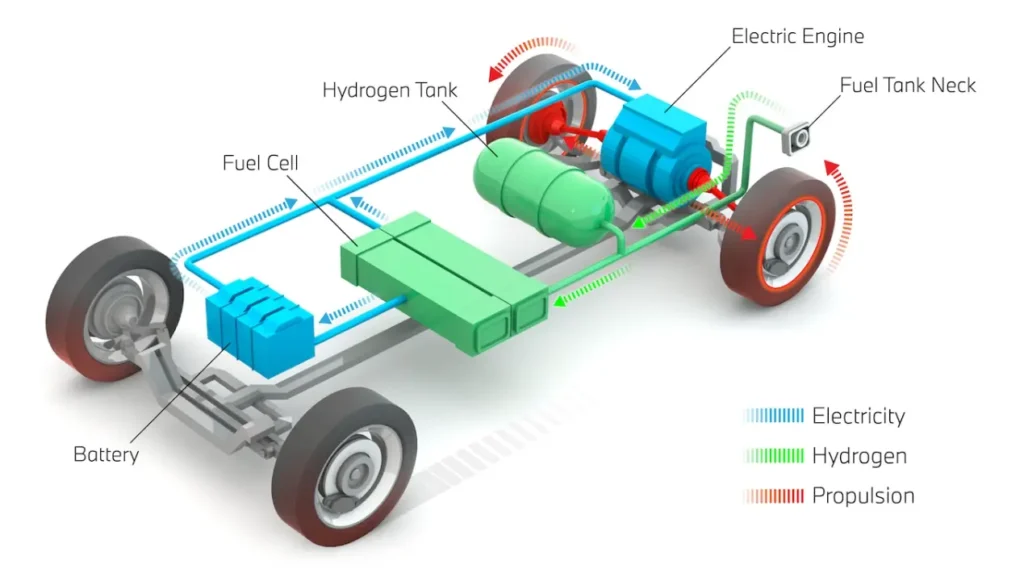
Figure 6- The Schematic of Hydrogen Fuel Cell Cars
Here’s a breakdown of how Hydrogen Fuel Cell cars works:
- Hydrogen Storage: Hydrogen is stored in pressurized tanks within the car.
- Fuel Cell Stack: The heart of the system, where hydrogen reacts with oxygen in the air.
- Electrochemical Reaction: Hydrogen molecules are split into protons and electrons at the anode. Protons pass through the electrolyte to the cathode, while electrons travel through an external circuit, generating electricity to power the motor.
- Oxygen Supply: Oxygen from the air is fed to the cathode to combine with the protons and electrons, forming water as the only emission.
- Electric Motor: The electricity generated drives the motor, propelling the car forward.
Hydrogen fuel cells advantages include zero emissions (only water vapor), fast refueling, and long driving range. These, making hydrogen fuel cell cars a promising alternative to, where Hydrogen fuel cell efficiency is higher than battery electric vehicles.
Fuel Cell Simulation in ANSYS Fluent
At CFD LAND, we explore not only the theory behind fuel cells but also how to analyze and optimize those using Computational Fluid Dynamics (CFD). One of the most widely studied systems is the PEM fuel cell, known for its use in vehicles and portable devices (Fig.7). Using ANSYS Fluent, we can build detailed 3D or 2D models to simulate the electrochemical and fluid transport processes occurring in the cell.
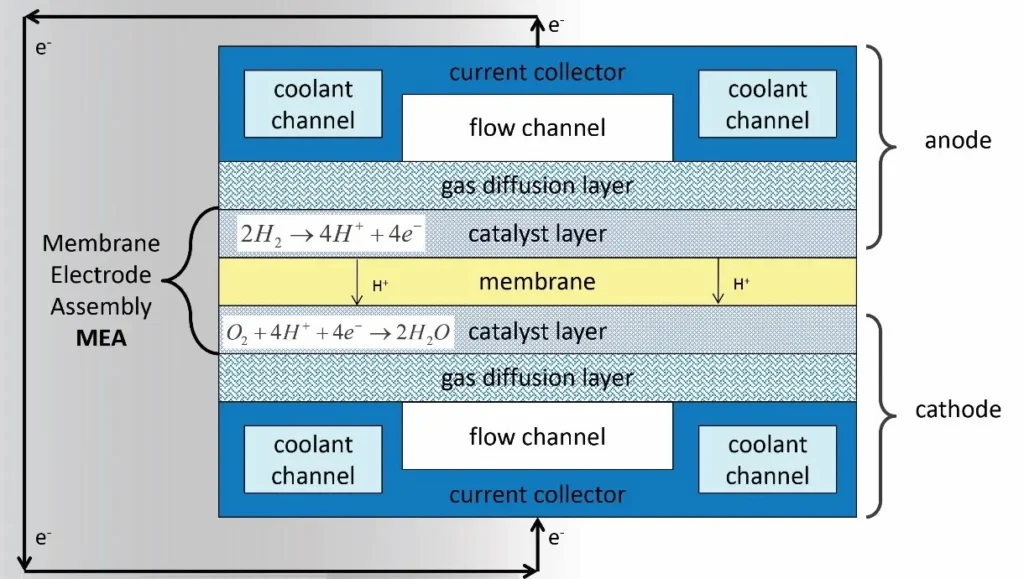
Figure 7-ANSYS Fluent: PEM Fuel Cell (PEMFC) Model Schematic
If you want to learn PEM fuel cell simulation step-by-step in Fluent, Check out our popular package (Fig.8). This tutorial demonstrates the basic workflow used to set up a simulation describing a Polymer Electrolyte Membrane Fuel Cell (PEMFC) in ANSYS Fluent (Fig.8).

Figure 8- Proton Exchange Membrane Fuel Cell (PEMFC) CFD Simulation | Numerical Paper Validation
This hands-on tutorial walks you through geometry, meshing, model setup, and validation against a published research paper (Fig.9)—ideal for students, researchers, and professionals in fuel cell R&D.
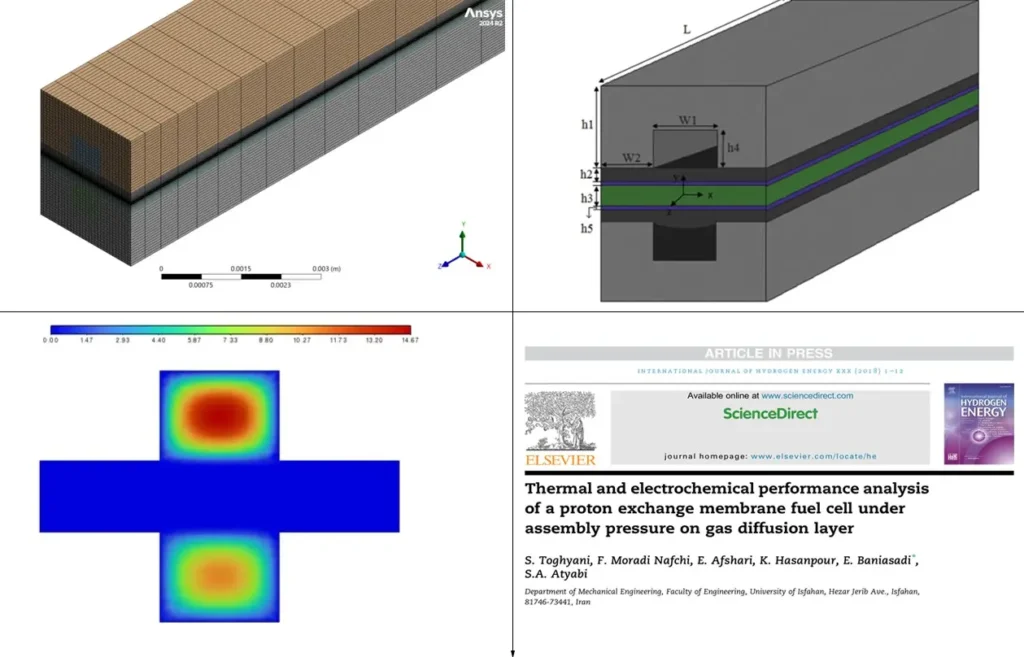
Figure 9- Thermal and electrochemical performance analysis of a proton exchange membrane fuel cell under assembly pressure on gas diffusion layer [5]
Simulations like this not only enhance our understanding of how hydrogen fuel cells behave in practice but also help in optimizing hydrogen fuel cell efficiency and improving real-world design for commercial applications.
How to Enable the Fuel Cell Module in ANSYS Fluent
By default, the Fuel Cell module is not activated in ANSYS Fluent. To use it, you need to manually enable it using the Text User Interface (TUI). Follow these steps:
- Open ANSYS Fluent and proceed to the main setup window.
- In the command line area at the bottom of the Fluent window, type the following command:
/define/models/addon-module
!!!Note that, the Fuel Cell Module activation process has changed in newer versions of ANSYS Fluent. If you are using the older version you should type: /define/models/fuel-cell yes“
- Then, a list of available add-on modules will appear (Fig.10). Find the number corresponding to Fuel Cell in the list.
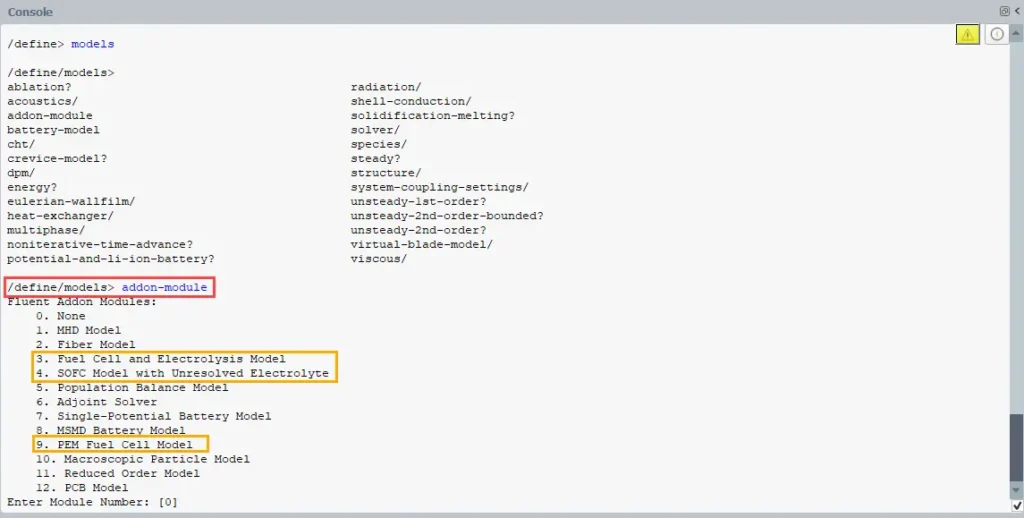
Figure 10- The process of activating the Fuel Cell Model in the command line area of ANSYS Fluent
4. Type the number (e.g., 3) and press Enter to activate it.
5. Once activated, you will find the Fuel Cell option available under the Models You can now configure the fuel cell settings e.g., for PEMFC model (Fig.11).
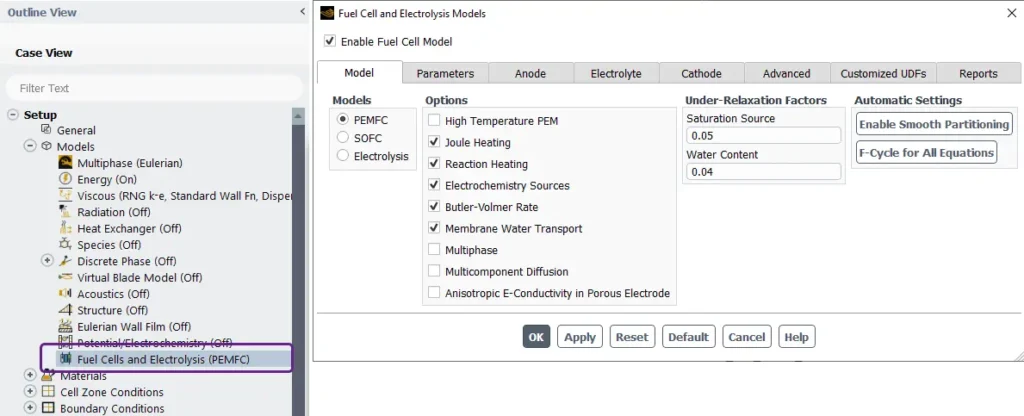
Figure 11- Schematic of the Fuel Cells and Electrolysis Models panel in ANSYS Fluent
Fuel Cells and Electrolysis Models interface in ANSYS Fluent
This figure 11 shows the setup interface of a simulation in ANSYS Fluent, for simulating the Fuel Cells and Electrolysis Model (PEMFC).
Model Tab:
- Models: Three options are available:
- PEMFC(Proton Exchange Membrane Fuel Cells) is selected.
- SOFC(Solid Oxide Fuel Cells).
- Electrolysis.
Options:
-
- Features like High Temperature PEM, Joule Heating, Reaction Heating, Electrochemistry Sources, Membrane Water Transport, and others can be toggled on/off based on the simulation requirements.
Under-Relaxation Factors:
-
- Parameters for Saturation Source (default: 0.05) and Water Content (default: 0.04) are adjustable for numerical stability during iterations.
Automatic Settings:
-
- Enable Smooth Partitioning: Helps optimize mesh handling and computational efficiency.
- F-Cycle for All Equations: Ensures robust convergence during iterative solving.
Other Tabs at the Top:
The interface allows switching between tabs for further configurations:
- Model(Current tab).
- Parameters: Likely for global and specific simulation settings.
- Anode, Electrolyte, Cathode: Detailed material and boundary condition definitions for each component.
- Advanced: Additional solver or model refinements.
- Customized UDFs: User-defined functions for custom behavior.
- Reports: Post-processing and analysis outputs.
The PEM Fuel Cell Model setup in ANSYS Fluent
A Proton Exchange Membrane Fuel Cell (PEMFC) is a type of fuel cell that converts chemical energy directly into electrical energy through electrochemical reactions. The PEMFC model in ANSYS Fluent provides a comprehensive framework to simulate and analyze the physical, chemical, and thermal processes occurring within the fuel cell. In the Model tab shown here (Fig.12), you can enable PEMFC simulations and customize key options such as:
- Joule Heating, Reaction Heat, and Electrochemistry Sources for thermal and reaction modeling.
- Butler-Volmer Rate and Multi-Component Diffusion for accurate electrochemical and species transport analysis.
- Membrane and GDL properties like Anisotropic Proton Conductivity and Liquid Phase Dynamics for detailed water and ion transport.
Additional tabs allow configuring parameters, electrode designs (Anode/Cathode), membrane properties (Electrolyte), electrical system setup (Electrical Tabs), advanced customization (Advanced), and scripting with UDFs. Each tab helps tailor the simulation to specific design requirements.
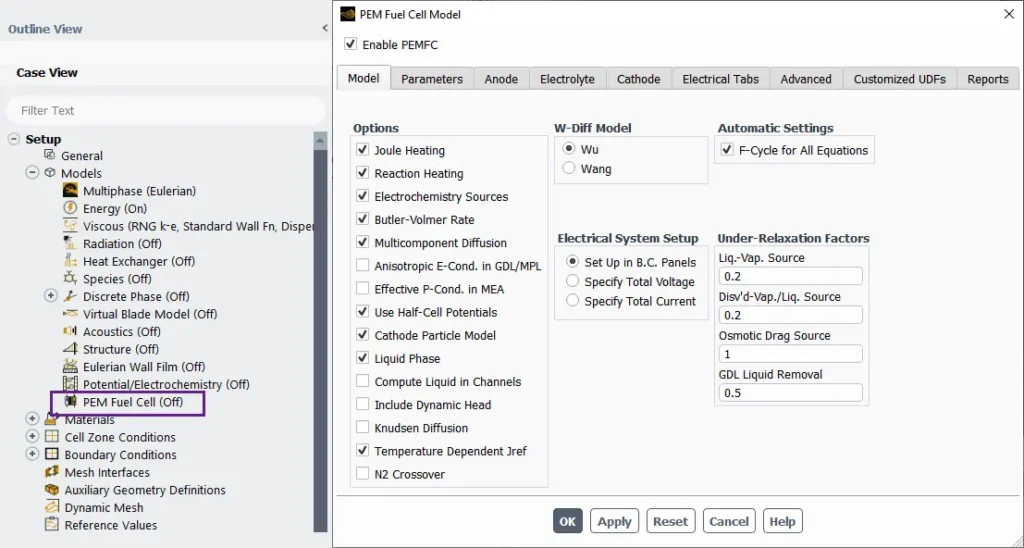
Figure 12- Schematic of the PEM Fuel Cell Model panel in ANSYS Fluent
The SOFC (Solid Oxide Fuel Cell) Model setup in ANSYS Fluent
The SOFC (Solid Oxide Fuel Cell) Model setup in ANSYS Fluent provides a comprehensive framework for simulating fuel cells with unresolved electrolyte regions (Fig.13).
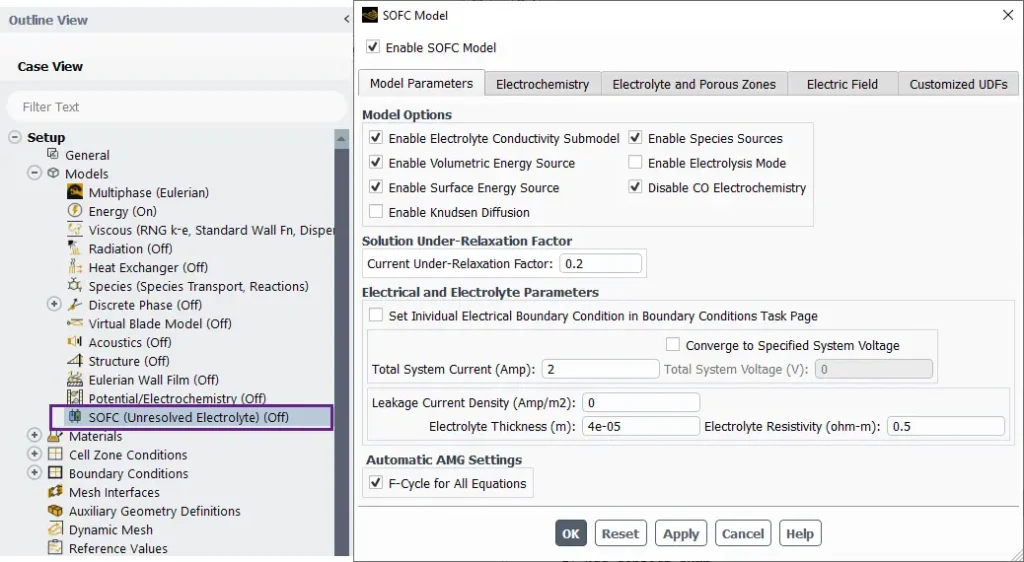
Figure 13- Schematic of the SOFC Model panel in ANSYS Fluent
This model is ideal for studying electrochemical reactions, heat transfer, and fluid flow in SOFC systems. Here’s an overview of the key features in the interface. In the Model Options tab shown here, there are some options such as:
- Enable Volumetric Energy Source: Includes heat generation in the electrolyte and electrode regions.
- Enable Surface Energy Source: Activates reaction energy sources at catalytic surfaces.
- Solution Under-Relaxation Factor: Specifies the relaxation factor for the current solution to ensure numerical stability during convergence.
- F-Cycle for All Equations: Optimizes the algebraic multigrid (AMG) solver to improve convergence for all equations.
Additional Tabs
- Electrochemistry: Configure reaction kinetics and electrode properties.
- Electrolyte and Porous Zones: Set up electrolyte behavior and porous media for the fuel cell.
- Electric Field: Define electric field properties and coupling.
- Customized UDFs: Add user-defined functions for advanced customization.
Conclusion
As the world shifts toward sustainable energy, fuel cells stand out as a clean, efficient, and scalable solution. Technologies like hydrogen fuel cells and PEM fuel cells offer promising alternatives to traditional combustion engines and power systems. Their advantages in zero-emission performance, especially in vehicles and portable power, make them essential for future energy systems. With the support of CFD simulations in ANSYS Fluent, researchers and engineers can optimize fuel cell designs, improve efficiency, and reduce development time. As infrastructure improves, fuel cells are set to play a leading role in the global clean energy transition. At CFD LAND, we utilize ANSYS Fluent to simulate and optimize fuel cell performance, advancing hydrogen fuel cell efficiency and commercial application designs which is ideal study for students, researchers, and professionals in fuel cell R&D.
FAQs
- What are fuel cells?
Fuel cells are electrochemical devices that convert chemical energy from a fuel, typically hydrogen, directly into electricity, heat, and water with high efficiency and low emissions.
- How do fuel cells work?
Fuel cells work by splitting hydrogen at the anode into protons and electrons. The electrons generate electricity through an external circuit, while protons move through a membrane to the cathode, where they combine with oxygen to form water.
- What is the difference between fuel cells vs batteries?
Unlike batteries, fuel cells don’t store energy but continuously generate electricity as long as fuel is supplied. Fuel cells offer longer operation and quicker refueling, while batteries are more common and currently easier to recharge.
- What are the types of fuel cells?
Common types include Proton Exchange Membrane Fuel Cells (PEMFC), Solid Oxide Fuel Cells (SOFC), and Alkaline Fuel Cells (AFC), each suited for specific applications.
- What are the advantages and disadvantages of hydrogen fuel cells?
- Advantages: Zero emissions, quick refueling, and high efficiency.
- Disadvantages: High cost, limited hydrogen infrastructure, and challenges in hydrogen storage.
- Where are fuel cells used?
Fuel cells applications include electric vehicles, backup power systems, portable chargers, drones, and even submarines and space shuttles.
- What challenges do fuel cells face?
Challenges include high costs, hydrogen production and storage issues, and the need for infrastructure development for widespread adoption.
Reference
[1] Armenta-Déu, C. (2025). A Fuel Cell Control System for Performance Improvement under Variable Atmospheric Pressure Conditions. Recent Progress in Science and Engineering, 1(1), 1-20.
[2] Ramadhani, F., Hussain, M. A., & Mokhlis, H. (2019). A comprehensive review and technical guideline for optimal design and operations of fuel cell-based cogeneration systems. Processes, 7(12), 950.
[3] Sebbani, I., Ettouhami, M. K., & Boulakhbar, M. (2025). Fuel Cells: A Technical, Environmental, and Economic Outlook. Cleaner Energy Systems, 100168.
[4] Samsudin, A. M., Bodner, M., & Hacker, V. (2022). A brief review of poly (vinyl alcohol)-based anion exchange membranes for alkaline fuel cells. Polymers, 14(17), 3565.
[5] Toghyani, S., Nafchi, F. M., Afshari, E., Hasanpour, K., Baniasadi, E., & Atyabi, S. A. (2018). Thermal and electrochemical performance analysis of a proton exchange membrane fuel cell under assembly pressure on gas diffusion layer. International Journal of Hydrogen Energy, 43(9), 4534-4545.